
New Hope: Malaria Drug for Infants
By Darius Spearman (africanelements)
Support African Elements at patreon.com/africanelements and hear recent news in a single playlist. Additionally, you can gain early access to ad-free video content.
Malaria Treatment Breakthrough for Babies
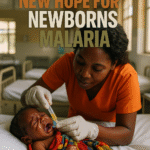
A significant breakthrough has occurred in the global fight against malaria, offering renewed hope for the most vulnerable among us: newborn babies and young infants. For the first time ever, a malaria treatment specifically designed and approved for this age group has been developed and given the green light. This new medication, known as Coartem Baby or Riamet Baby, is the result of a collaborative effort between the pharmaceutical company Novartis and the Medicines for Malaria Venture (MMV), a non-profit organization based in Switzerland (bbc.com).
Until this development, there was a critical gap in malaria treatment. Infants weighing less than 4.5 kilograms had no approved medication tailored to their specific needs (novartis.com). This meant that healthcare providers were forced to use formulations intended for older children, which carried serious risks. Administering drugs designed for larger bodies to tiny infants could lead to overdose and toxicity, making treatment a dangerous balancing act (novartis.com). Furthermore, malaria vaccines, which offer a crucial layer of protection, are also not approved for the youngest babies, leaving them even more exposed to the disease (novartis.com).
Addressing a Critical Need
The approval of Coartem Baby directly addresses this critical unmet need, providing a safe and effective treatment option for the most vulnerable population. Novartis CEO Vas Narasimhan expressed the company's pride, stating that they are “proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve” (reuters.com, novartis.com). This new drug is specifically formulated to help babies and very young children infected by malaria (bbc.co.uk).
Malaria poses a particularly severe threat to young children and babies. Historically, there has not been a treatment specifically designed to meet their unique physiological needs (bbc.co.uk). This new development fills that void, offering a tailored solution that minimizes the risks associated with off-label drug use. The impact of this approval is expected to be profound, especially in regions where malaria is endemic and infant mortality rates due to the disease are tragically high.
Malaria's Impact on Babies
Millions of babies are born into areas where malaria is a constant threat, particularly across Africa. This highlights the urgent necessity for this new treatment. Every year, approximately 30 million babies are born in African regions where malaria is a significant risk (novartis.com). In 2023 alone, an estimated 36 million pregnancies occurred in 33 African countries where malaria is widespread (euronews.com).
The danger extends beyond direct exposure. About one in three mothers in these regions were infected with malaria during their pregnancy, which significantly increases the risk of passing the disease to their newborns (euronews.com). A large survey conducted across West Africa revealed that malaria infections in infants younger than six months old ranged between 3.4% and 18.4% (novartis.com). These statistics underscore the immense burden of malaria on the youngest members of these communities and the critical need for effective interventions like Coartem Baby.
Malaria Cases in Uganda (2023)
Understanding Coartem Baby
Coartem Baby, also known as Riamet Baby in certain regions, is a groundbreaking malaria treatment developed by Novartis in collaboration with the Medicines for Malaria Venture (MMV) (bbc.com). It stands as the first approved malaria treatment specifically for newborns and young infants (novartis.com). This medication is a combination of two powerful antimalarial drugs: artemether and lumefantrine. This combination therapy is crucial because it targets the malaria parasite at different stages of its life cycle, making it highly effective and helping to prevent the development of drug resistance.
The significance of this drug lies in its tailored formulation. Before Coartem Baby, infants weighing less than 4.5 kilograms had no approved malaria treatment (novartis.com). This created a dangerous treatment gap, forcing medical professionals to administer drugs intended for older children. Such practices carried a significant risk of overdose and toxicity, as the dosages were not appropriate for the delicate systems of newborns (novartis.com). Coartem Baby addresses this by providing precise, infant-friendly dosing, ensuring both safety and efficacy for the smallest patients.
Why Malaria Harms Infants
Malaria is particularly dangerous for young children and babies (bbc.co.uk). While it was once believed that newborns were largely protected from malaria due to maternal immunity, this understanding has evolved. Experts now recognize that the burden of malaria in very young children is substantial, even if their symptoms might present differently than in older individuals (washingtonpost.com). The immune systems of newborns are still developing, making them highly vulnerable to infections. Even low levels of malaria parasites can lead to severe complications and, tragically, death in these very small children (washingtonpost.com).
The previous lack of a specific treatment for infants under 4.5 kilograms exacerbated this vulnerability. Without a tailored medication, healthcare providers faced immense challenges in safely and effectively treating these tiny patients. The development of Coartem Baby means that medical professionals now have a tool specifically designed to combat malaria in this fragile population, offering a much-needed lifeline and reducing the risks associated with inappropriate dosing.
Weight Thresholds and Safety
The specific weight thresholds, particularly the 4.5-kilogram mark, are crucial for understanding the significance of Coartem Baby. Until now, there was no approved malaria treatment for infants weighing less than 4.5 kilograms, which created a dangerous gap in care (novartis.com). This meant that infants in this weight category were often treated with malaria formulations intended for older children. Such practices carried a significant risk of overdose and toxicity because the dosages were not calibrated for their smaller body masses and developing physiological systems (novartis.com).
The new drug, Coartem Baby, is specifically formulated to address these weight-based dosing challenges. By providing a precise and safe dosage for infants within this critical weight range, it eliminates the need for risky off-label treatments. This ensures that the medication is both effective in combating the malaria parasite and safe for the delicate systems of newborns, preventing potential harm from improper dosing.
Maternal Immunity and Newborns
Maternal immunity was once widely believed to provide comprehensive protection to newborn babies from malaria (washingtonpost.com). This traditional understanding suggested that antibodies passed from mother to child during pregnancy would shield the infant from infection in their early months. However, current scientific understanding has evolved, revealing that this protection is not absolute. While some level of immunity may be conferred, it is now recognized that the burden of malaria in very young children is significant, even if they present with different symptoms compared to older individuals (washingtonpost.com).
This shift in understanding underscores the urgent need for direct interventions like Coartem Baby. Despite any maternal antibodies, newborns remain vulnerable to malaria, and even low levels of parasites can lead to severe disease and death. The development of a specific treatment for this age group acknowledges that maternal immunity alone is insufficient to fully protect them, and active treatment is often necessary to save lives.
Exclusion from Clinical Trials
Historically, young babies have been rarely included in clinical trials for antimalarial agents (novartis.com). This exclusion has had significant implications for treatment development. Because infants were not part of these crucial studies, there has been a severe lack of data on how malaria affects this vulnerable population and how different drugs interact with their developing bodies. This absence of information directly contributed to the treatment gap, as pharmaceutical companies lacked the necessary data to develop and gain approval for formulations specifically tailored for newborns and young infants (novartis.com).
The development and approval of Coartem Baby represent a monumental step forward, as it involved overcoming the challenges of conducting trials in this sensitive population. This achievement means that, for the first time, there is a clinically proven and approved treatment based on data gathered specifically for the youngest malaria patients, ensuring their safety and efficacy.
Malaria's Impact on Pregnancies in Africa (2023)
Malaria's Impact on Pregnancies in Africa (2023)
Malaria Vaccines and Infants
While the approval of Coartem Baby marks a significant advance in treating malaria, it is important to note that malaria vaccines are currently not approved for the youngest babies (novartis.com). This means that for infants, prevention strategies are still limited, and the focus remains heavily on treatment once the disease is contracted. Malaria vaccines can typically be administered to children starting around five months of age, and they require a series of four doses, according to the World Health Organization (washingtonpost.com).
The absence of a vaccine for the earliest months of life underscores the critical importance of a safe and effective treatment like Coartem Baby. It ensures that even before they are eligible for vaccination, the most vulnerable infants have a fighting chance against this deadly disease. This highlights the need for a multi-faceted approach to malaria control, combining both treatment and prevention strategies as they become available for different age groups.
Safety and Efficacy of Coartem Baby
The approval of Coartem Baby as the first clinically proven malaria treatment for newborns and young infants is a testament to its safety and efficacy (novartis.com). While specific details on its full safety profile, potential side effects, or precise efficacy rates are not extensively detailed, the primary focus of its approval is to address the critical treatment gap for this vulnerable population. Before this development, infants were treated with formulations meant for older children, which carried a significant risk of overdose and toxicity (novartis.com).
The very fact that Coartem Baby has received regulatory approval signifies that it has undergone rigorous testing to ensure it is safe and effective for its intended use. This new drug represents a substantial improvement over previous practices, providing a tailored and safer option for treating malaria in the smallest patients, thereby reducing the dangers associated with inappropriate dosing.
Accessibility and Rollout
The rollout of Coartem Baby is expected to begin in African countries, where malaria is most prevalent, within the next few weeks (bbc.co.uk). To ensure timely access, individual countries are expected to approve the use of the treatment within 90 days after it is submitted for regulatory approval (washingtonpost.com). While Novartis is involved in this initiative, specific details regarding the distribution mechanisms, affordability for families, or the readiness of healthcare infrastructure in various African countries are not yet fully outlined.
Despite these missing details, the commitment to a rapid rollout in high-burden areas is a positive sign. The goal is to get this life-saving medication to the infants who need it most as quickly as possible. This widespread distribution will be crucial in making a tangible difference in the fight against infant malaria across the continent.
Infant Malaria Infection Rates in West Africa
Implementation Timeline and Regulatory Approvals
The expected rollout of Coartem Baby in African countries is anticipated “within the next few weeks” (bbc.co.uk). This rapid timeline is crucial given the urgent need for the treatment. To facilitate timely access, individual countries are expected to approve the use of the treatment within 90 days after it is submitted for regulatory approval (washingtonpost.com). This expedited approval process aims to minimize delays in getting the drug to the infants who desperately need it.
While the general timeline is encouraging, a detailed schedule or specific information on regulatory approvals for all targeted African countries beyond Ghana is not yet available. However, the commitment to a swift and widespread deployment underscores the global effort to combat infant malaria effectively.
Long-Term Impact and Integration
The approval of Coartem Baby represents a monumental step forward in treating malaria in newborns and young infants. However, the current information does not extensively discuss its potential long-term impact on malaria mortality rates in infants or how it will integrate with existing malaria control programs. The immediate focus is on addressing the critical treatment gap for this vulnerable age group (novartis.com).
Despite this, the introduction of a safe and effective treatment for infants is expected to have a significant positive effect on overall public health. It will likely reduce severe cases and deaths among the youngest patients, thereby contributing to broader malaria control efforts. As the drug becomes more widely available, its impact on infant mortality and its role within comprehensive malaria strategies will become clearer.
Alternative Treatments and Risks
Before the approval of Coartem Baby, infants weighing less than 4.5 kilograms were treated with malaria formulations intended for older children (novartis.com). The primary risk associated with this practice was a significant increase in the chance of overdose and toxicity. These older formulations were not designed for the delicate physiology of newborns, making accurate and safe dosing extremely challenging. This often led to situations where infants received either too much medication, causing harmful side effects, or too little, rendering the treatment ineffective.
Coartem Baby represents a profound improvement by providing a clinically proven and appropriately dosed treatment specifically for this vulnerable group. It eliminates the need for risky off-label prescribing, ensuring that infants receive the precise amount of medication needed to combat malaria without endangering their health. This advancement is a game-changer for infant malaria care.
Dosage Form and Administration
Coartem Baby is described as “infant-friendly” and “easy to take” (bbc.co.uk). While these descriptions are reassuring, the provided information does not specify its exact dosage form, such as whether it is a syrup, dispersible tablets, or another formulation. Similarly, detailed instructions on its administration method are not included. This information would be crucial for caregivers and healthcare workers to ensure proper and effective delivery of the medication.
However, the emphasis on its “infant-friendly” nature suggests that it is designed to be easily administered to very young children, likely in a form that can be mixed with food or liquid, or a small, easy-to-swallow tablet. This user-friendly design is vital for ensuring compliance and successful treatment outcomes in real-world settings.
Addressing Drug Resistance
The provided information focuses primarily on the approval of Coartem Baby as the first malaria treatment for newborns and young infants, highlighting its role in addressing a significant treatment gap (novartis.com). However, it does not contain specific details regarding whether this new treatment directly addresses concerns about malaria parasite resistance to existing drugs. Malaria parasites have a known ability to develop resistance to antimalarial medications, making the development of new and effective treatments a continuous challenge.
Given that Coartem Baby is a combination therapy of artemether and lumefantrine, it inherently offers a degree of protection against resistance. Combination therapies are generally more effective at preventing resistance because the parasites would need to develop resistance to multiple drugs simultaneously, which is less likely. While explicit details on its resistance profile are not provided, the use of a proven combination therapy is a positive indicator in the ongoing fight against drug-resistant malaria.
Broader Public Health Context
The approval of Coartem Baby is a monumental step in addressing malaria in newborns and young infants. This development fills a critical gap in treatment options for the most vulnerable population. However, the provided information primarily focuses on this specific treatment and does not extensively detail how it integrates into the broader public health context of malaria control. Comprehensive malaria control involves a multifaceted approach that includes prevention strategies, vector control (like insecticide-treated bed nets), and treatments for older children and adults.
While the new drug is a vital tool, it is one piece of a larger puzzle. The fact that malaria vaccines are not approved for the youngest babies (novartis.com) further emphasizes the continued need for integrated strategies. This includes ongoing research into new prevention methods, strengthening healthcare systems to deliver treatments effectively, and educating communities on malaria prevention. Coartem Baby will undoubtedly save lives, but its full impact will be realized when combined with existing and future public health initiatives to eradicate malaria.
ABOUT THE AUTHOR
Darius Spearman has been a professor of Black Studies at San Diego City College since 2007. He is the author of several books, including Between The Color Lines: A History of African Americans on the California Frontier Through 1890. You can visit Darius online at africanelements.org.
