
New USAID Study Expands HIV Prevention Options for Women and Girls in Africa
By Darius Spearman (africanelements)
Support African Elements at patreon.com/africanelements and hear recent news in a single playlist. Additionally, you can gain early access to ad-free video content.
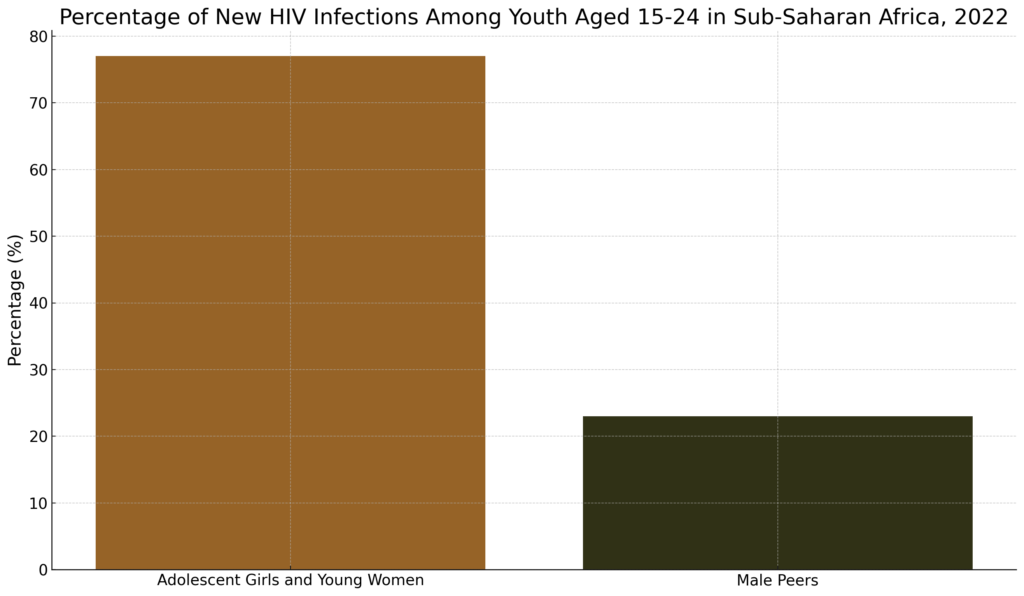
The HIV/AIDS epidemic continues to disproportionately impact adolescent girls and young women in sub-Saharan Africa. Despite progress, this vulnerable group accounted for a staggering 77% of new HIV infections among youth aged 15-24 in the region in 2022. (UNAIDS, 2022; Bor et al., 2022) Moreover, adolescent girls and young women in sub-Saharan Africa were more than three times as likely to acquire HIV compared to their male peers last year. (UNAIDS, 2022)
To address this alarming disparity, the U.S. Agency for International Development (USAID) is funding an innovative project called “Maximizing Options to Advance Informed Choice for HIV Prevention” (MOSAIC). (FHI 360, n.d.) This five-year global initiative aims to accelerate the introduction and scale-up of new biomedical HIV prevention products specifically for adolescent girls, young women, and other women at substantial HIV risk.
Flagship CATALYST Study Tests New Prevention Products
MOSAIC’s flagship effort is the “Catalyzing Access to New Prevention Products to Stop HIV” (CATALYST) study. (PrEPWatch, n.d.) It will provide and assess an enhanced service delivery package offering oral pre-exposure prophylaxis (PrEP), the dapivirine vaginal ring, and injectable cabotegravir (CAB-PrEP) at PEPFAR/USAID sites in Kenya, Lesotho, South Africa, Uganda, and Zimbabwe.
“CATALYST will generate critical real-world evidence to support the wide-scale global implementation of these new prevention options,” said a USAID spokesperson. (PrEPWatch, n.d.)
Innovative New Prevention Methods
The dapivirine vaginal ring is a female-initiated HIV prevention product that can provide protection for 28 days. (WHO, 2021) Importantly, it is the first long-acting prevention method developed specifically for women. Clinical trials found the ring reduced HIV risk by up to 35% among consistent users.
In addition, injectable CAB-PrEP is another promising new option, providing eight weeks of continuous HIV protection with just six injections per year. (Unitaid, 2022) This long-acting method addresses challenges with oral PrEP adherence and stigma concerns.
Implementation Considerations
While rolling out these new products, the World Health Organization has highlighted key implementation needs, such as: (WHO, 2021)
- Providing comprehensive sexual health services alongside HIV prevention
- Ensuring women receive full information to make informed choices
- Addressing acceptability among key populations like sex workers
- Offering adherence support and demand creation activities
- Training healthcare providers on new prevention methods
- Gathering further safety data during pregnancy/breastfeeding
By proactively addressing these considerations, the MOSAIC consortium – led by FHI 360 with partners like Wits RHI and AVAC (FHI 360, n.d.) – aims to maximize access to and uptake of this multi-product HIV prevention market for women and girls in Africa.
About the author
Darius Spearman is a professor of Black Studies at San Diego City College, where he has been pursuing his love of teaching since 2007. He is the author of several books, including Between The Color Lines: A History of African Americans on the California Frontier Through 1890. You can visit Darius online at africanelements.org.
